
CERAMIC ENGINEERING - and Materials Science, more generally
The field of 'Materials' encompasses Ceramics, Metals, Polymers (plastics), and composites thereof
"Ceramics" encompasses both ceramics and glasses - these are strongly bonded and generally brittle materials, and can withstand high temperatures.
The challenge in answering the question "What are ceramics?" is that ceramics are used in a huge range of applications because they have useful thermal, mechanical, electrical, magnetic and optical properties. Some applications are:- Batteries and fuel cells - Li ion batteries have ceramic cathodes, and solid oxide fuel cells require mostly ceramic components
- Electronic subcomponents (capacitors, resonators, chip carriers, touchscreen glass and touchscreen electronics, power electronics substrates)
- Coatings or monolithic parts for high performance engines (jet engines and diesel engines) - because they can resist higher temperatures than the metal components
- Components for high performance cars and aircraft, perhaps even the wind leading edges on future hypersonic vehicles
- Catalyst and catalyst supports used in the petroleum and chemical industries
- All of the traditional applications like windows
Metals and Polymers
The study of "Materials Science" includes polymers, metals, ceramics, composites and perhaps biomaterials.Composites are made of 2 or more types of materials, in order to improve upon some property or function of the material or component. Examples include fiber reinforced plastic, ceramic-loaded polymers, ceramic-loaded metals, and fiber reinforced ceramics. Glass matrix composites are also of high industrial interest.
Let's have a look at a couple of examples of the applications of ceramics.......
Your cell phone is loaded with ceramic and glass components
 Really????
Really????
YES! Modern cell phones wouldn't be possible without ceramics, and even the 3G, 4G and soon-to-be 5G communications rely on ceramics.
Descriptions of some of the components are below, but let's think first about the operation of the cell phone:
..........it sends & receives microwave freqency waves to & from the cell tower......using ceramic components!
'Dielectric resonators' are ceramics based on oxides like BaTi4O9, or Ba[Zn1/3Ta2/3]O3. These are the "Talking Ceramics" and are needed for all wireless and GPS applications.
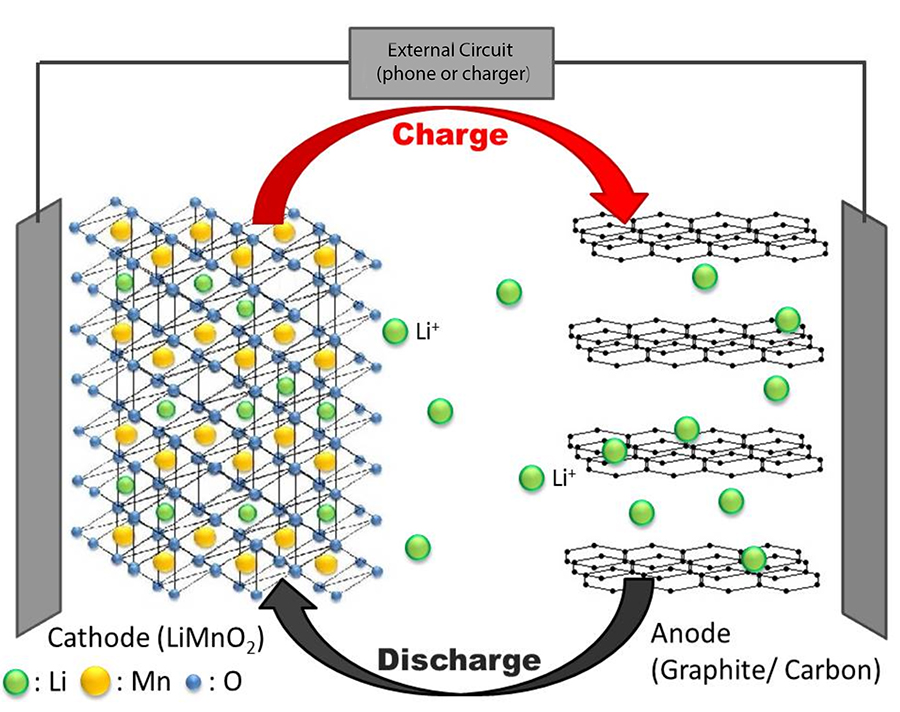
Cellphone Power: we need a good battery or all bets are off!
(The 2020 Nobel Prize in Chemistry went to the inventors of the Li ion battery)
The Li ion battery works by transfer of one electron at a time to/from the phone or charger by Li atoms converting to Li ions (which are missing one electron compared to the neutral atom).
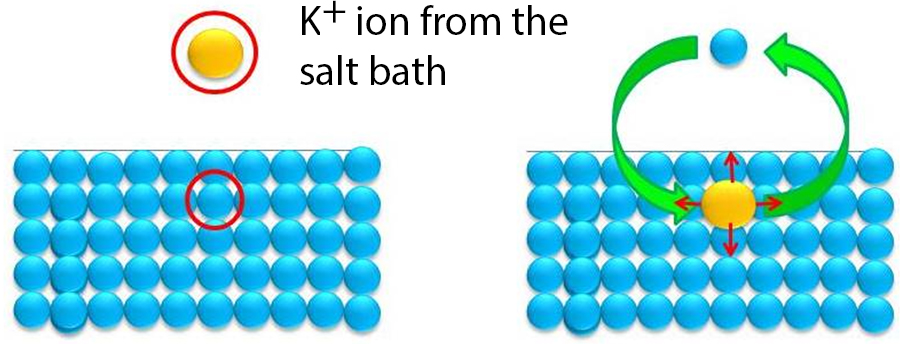
Cellphone screen...more complex than you might think...
Many phones use strengthened glass screens. Strengthening relies on builidng up a large compressive stress in the surface of the glass. This is done using a process called 'ion exchange,' wherein smaller Na+ ions in the glass surface are exchanged with larger K+ ions. This is usually done by submerging a panel of glass in a molten salt solution like KNO3.
 The back of the glass is
The back of the glass is
Electronic subcomponents make it work...
Some of the components are the resonators, capacitors, inductors and chip carriers. Ceramic piezoelectrics are used for the microphone, speakers and time keeping. Multilayer ceramic capacitors (MLCC) are of key importance in all electronics, and although they're small, it's a constant effort to make them even smaller. Your cell phone has at least 500 MLCCs! 
Solid oxide fuel cells directly convert fuel energy to electrons
 ....and so save lots of energy
....and so save lots of energy
SOFCs can be fueled by hydrogen, methane, natural gas, or even diesel. The simplest version uses hydrogen, but natural gas or methane SOFCs are currently running in the field.
These are composite devices: generally with ceramic electrodes and the key component is the ceramic electrolyte. However, they have metal gas manifolds, metal current collectors, and glass or compressive seals.
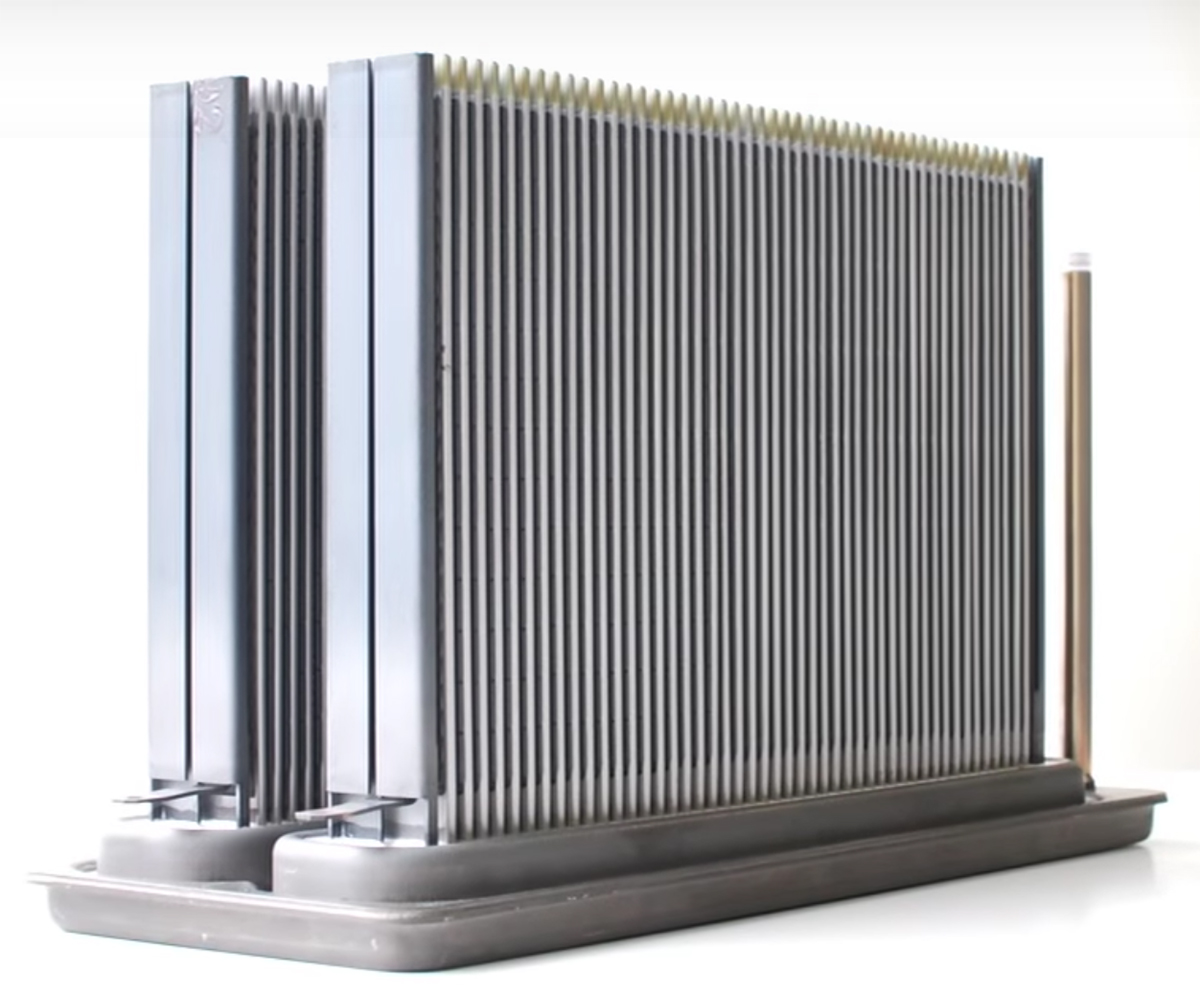
There is a spectacular video about SOFC technology made by Kyocera Corporation here:
[image at right is a Kyocera SOFC stack, without the ancillary parts. The vertical elements are each individual ceramic/metal SOFCs. From Kyocera, https://global.kyocera.com/tech/]
First step in operation: heat the fuel cell
 The ceramic electrolyte has a special property called "oxygen ion conductivity", meaning that oxygen, when it is split up into individual ions on the cathode side (of the form O2-), travels through to the opposite side of the fuel cell elecrolyte. Oxygen ions do this by skipping between adjacent vacant oxygen sites in the structure. That means, therefore, that we need a ceramic material with lots of missing oxygen, for example (Y0.08Zr0.92O1.96).
The ceramic electrolyte has a special property called "oxygen ion conductivity", meaning that oxygen, when it is split up into individual ions on the cathode side (of the form O2-), travels through to the opposite side of the fuel cell elecrolyte. Oxygen ions do this by skipping between adjacent vacant oxygen sites in the structure. That means, therefore, that we need a ceramic material with lots of missing oxygen, for example (Y0.08Zr0.92O1.96).
The anode and cathode are complex materials also, with the cathode based on (La,Sr)(Co,Fe)O3-x and the anode is a ceramic/metal composite of Y0.08Zr0.92O1.96 with Ni metal.
Add fuel and air.....
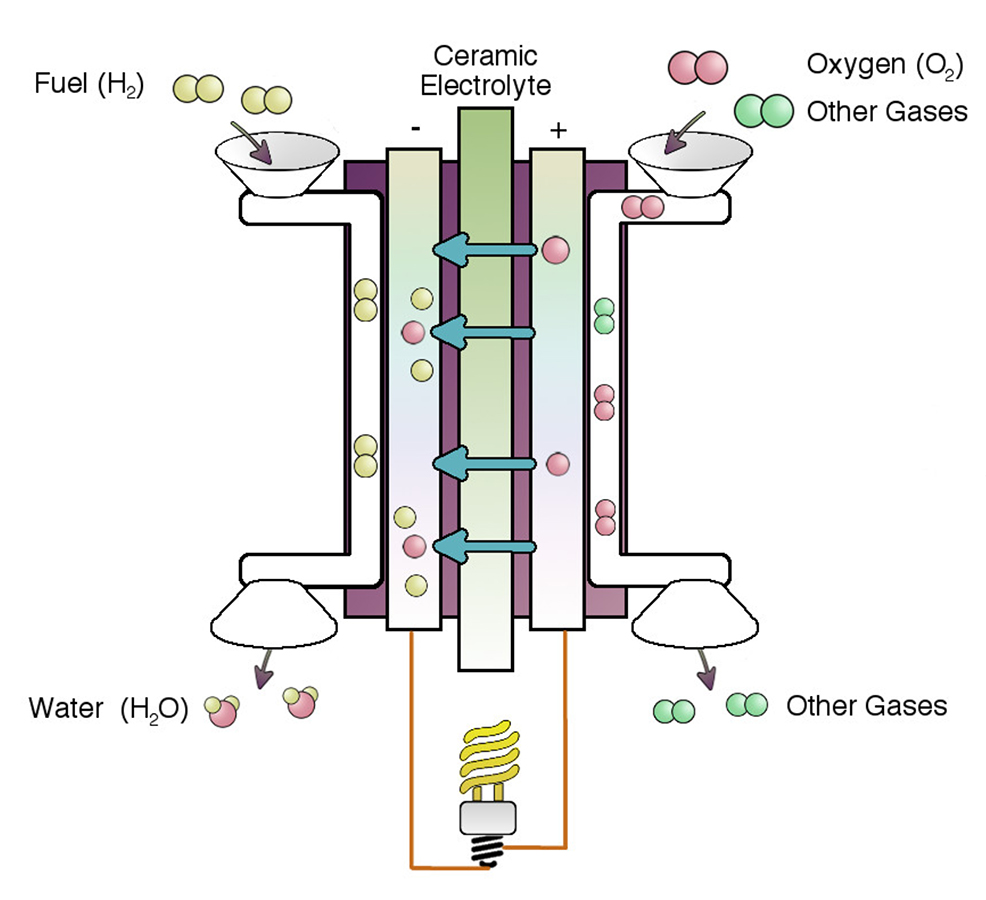 When the oxygen ion (with two extra electrons) arrives at the fuel side of the SOFC, the fuel is directly converted to exhaust, and the electrons are harvested into the external circuit. If we fuel the cell with H2, then the exhaust is simply H2O. Of course if we burn hydrocarbon fuels we still get carbon-containing exhaust streams......but......the efficiency is at least 20% higher than other technologies.
When the oxygen ion (with two extra electrons) arrives at the fuel side of the SOFC, the fuel is directly converted to exhaust, and the electrons are harvested into the external circuit. If we fuel the cell with H2, then the exhaust is simply H2O. Of course if we burn hydrocarbon fuels we still get carbon-containing exhaust streams......but......the efficiency is at least 20% higher than other technologies.
Why the high efficiency? Because there is no need to burn the fuel to heat water to make steam to turn a turbine. Instead we direclty capture the electrons transferred as the fuel is oxidized!!!
Pseudocapacitors bridge the gap between batteries and capacitors
Li ion batteries store a lot of energy (per weight) but charge and discharge slowly. Traditional capacitors store far less energy per mass but can charge and discharge in fractions of a second.
Intercalation pseudocapacitance: our area of interest
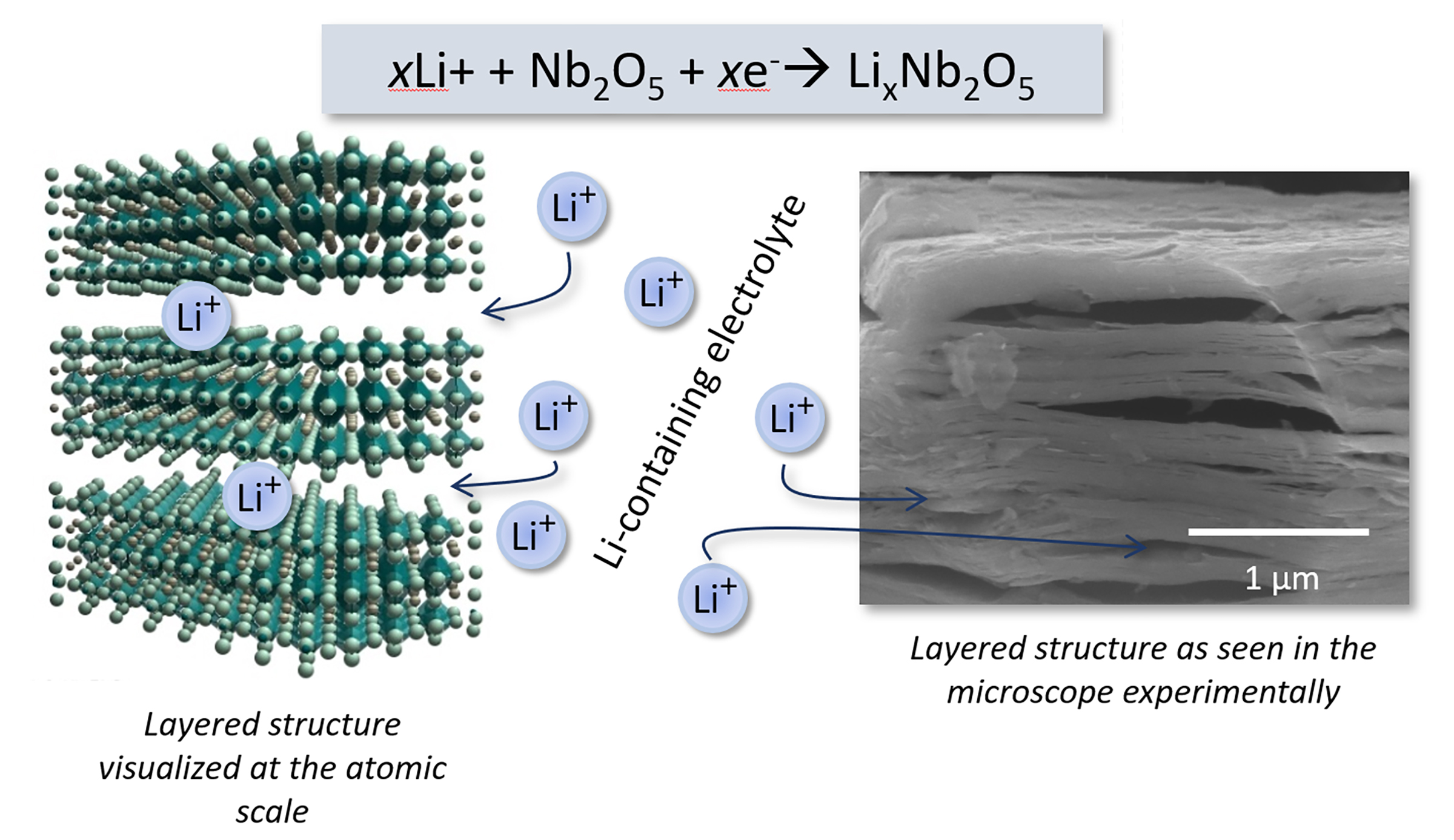
In a real device....
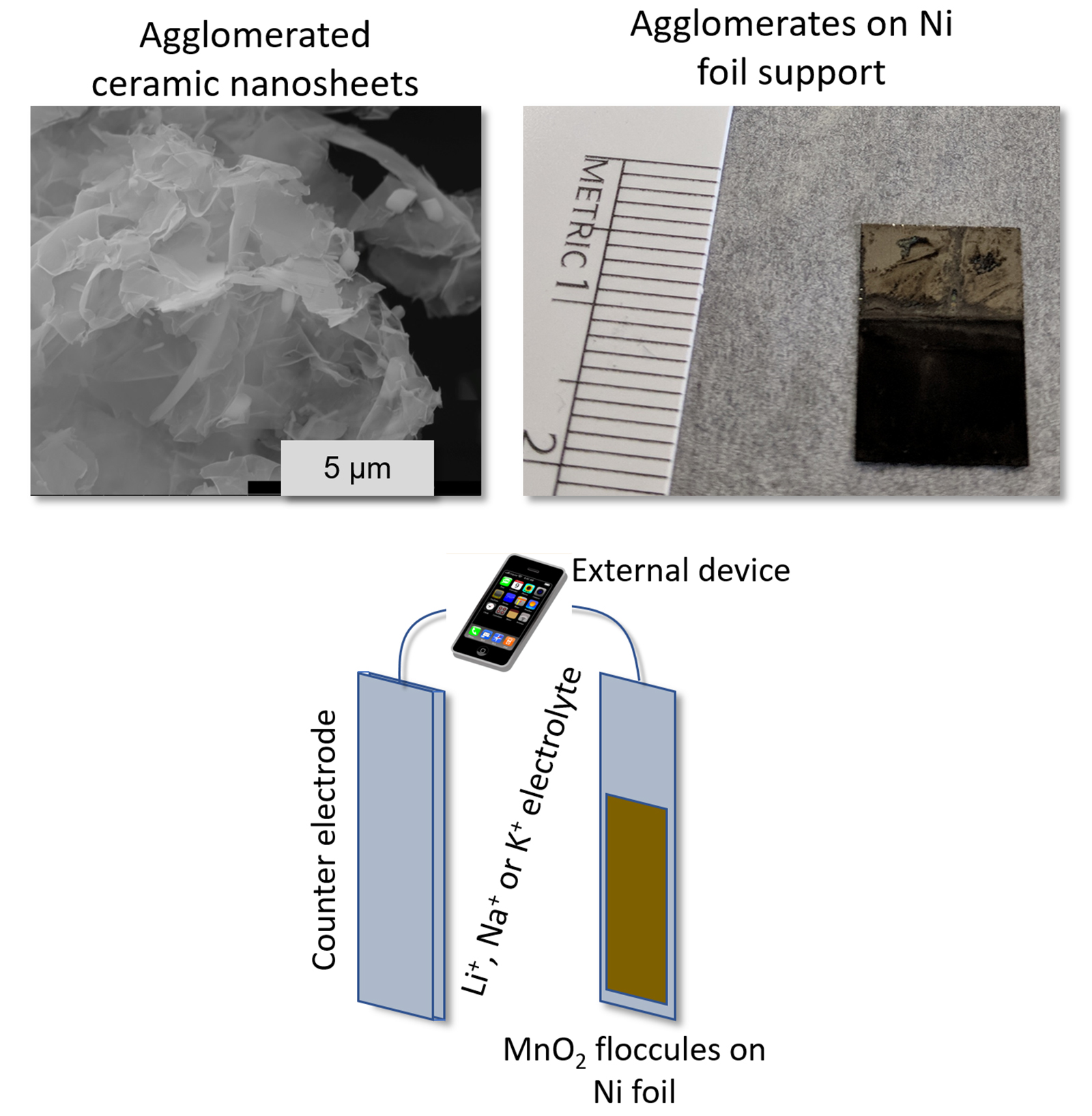 We make the layered ceramic materials into porous low density clusters via a process called 'flocculation' or 'gelation.'
We make the layered ceramic materials into porous low density clusters via a process called 'flocculation' or 'gelation.'
Next we attach those low-density layered oxide agglomerates to a conductive substrate, and immerse the whole thing in a liquid electrolyte which is just a solution of Li+, Na+, K+ or Rb+ in water.
The other electrode can be something like carbon, which can capture the ions as they move back and forth from the cathode and anode electrodes, providing about one electron to the external circuit for each ion transferred.